BIO-HELIX - QP019T-0100
COVID-19 RT-qPCR Detection Kit Plus
Size: 100 Rxns
In view of the joint global efforts of advancing collaborative research in diagnostics, therapeutics, and vaccination in
the fight against the COVID-19 (SARS-CoV-2) pandemic, Bio-Helix has specifically developed the LifeDireX
COVID-19 RT-qPCR Detection Kit Plus for human respiratory tract specimens. The kit is characterized by:
(1) High specificity for the N target markers as recommended by WHO and US CDC;
(2) Data obtained in less than 2 hours; and
(3) Compatible with standard RT-qPCR machines (ABI 7500, Bio-Rad CFX96, QuantStudio’s 7 Flex).
Application
》Gene Expression (mRNA) Analysis
》Copy Number Analysis
》SNP Genotype Analysis


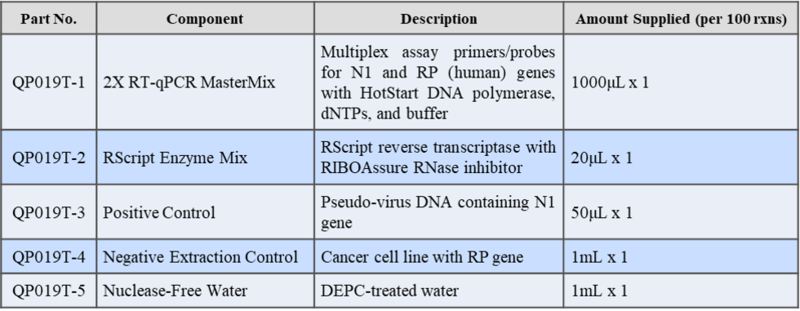
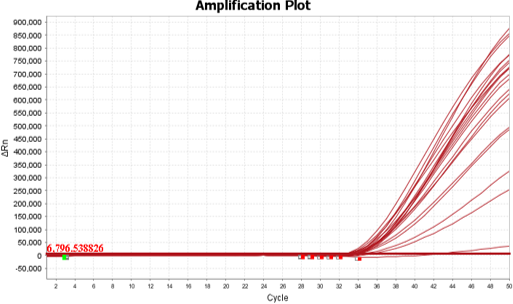
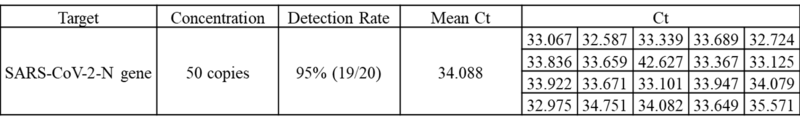
A study was performed to assess the performance of COVID-19 RT-qPCR Detection Kit Plus. A testing of 20 replicates at the tentative Limit of Detection (LoD) concentration was carried out with 50 copies/reaction at 95% detection rate. Samples were spiked SARS-CoV-2 RNA in pooled negative clinical nasopharyngeal swab matrix, then extracted with QIAamp Viral RNA Mini Kit and tested on QuantStudio™ 5 Real-Time PCR System. The synthetic SARS-CoV-2 RNA was obtained from Twist Biosciences (Assay Ready Control 14,EPI_ISL_710528).
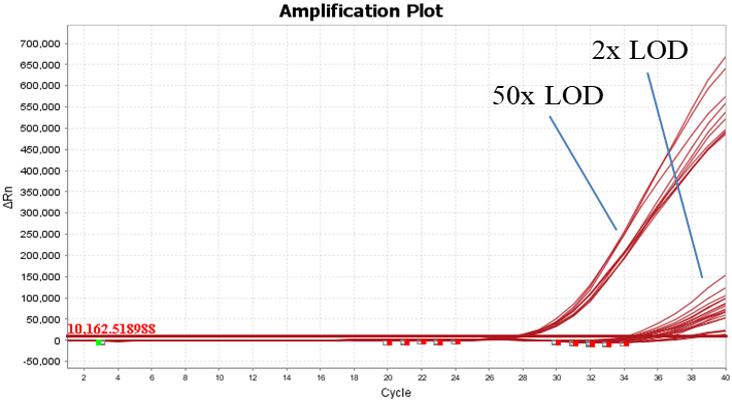
Figure 1. The result of specimens spiked with synthetic SARS-CoV-2 RNA.
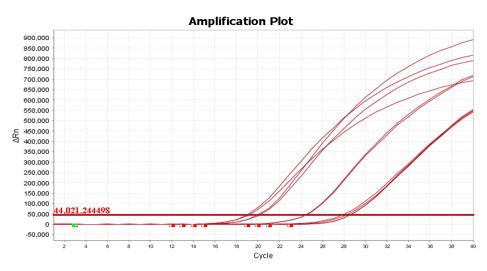
Figure 2. The result of clinical SARS-CoV-2 positive specimens.
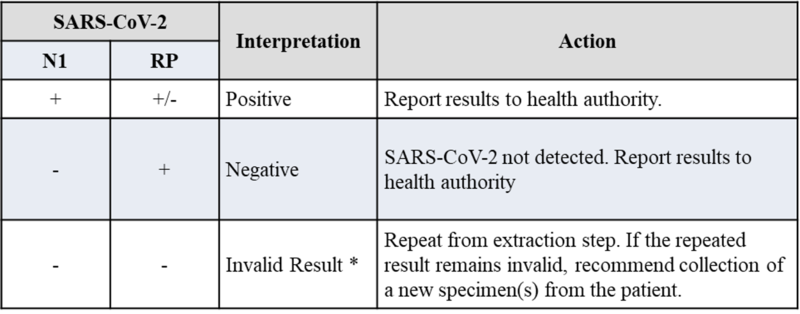
Table. Clinical Evaluation for nasopharyngeal swabs
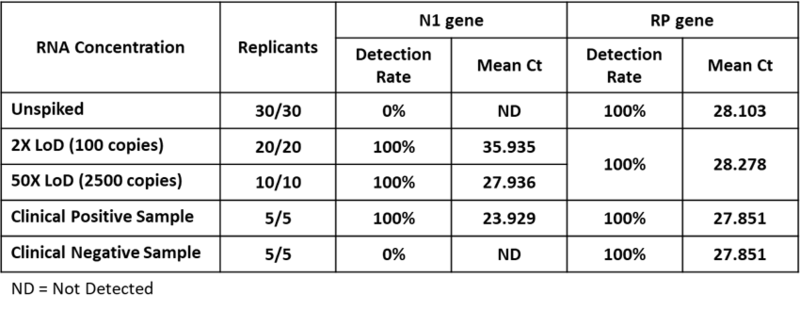
A study was performed to assess the clinical performance of COVID-19 RT-qPCR Detection Kit Plus. A testing of 20 replicates at low positive with 100 copies/reaction, 10 replicates at high positive with 2500 copies/reaction and 30 replicates were unspiked. Samples were spiked SARS-CoV-2 RNA in pooled negative clinical nasopharyngeal swab matrix, then extracted with QIAamp Viral RNA Mini Kit and tested on QuantStudio™ 5 Real-Time PCR System. The synthetic SARS-CoV-2 RNA was obtained from Twist Biosciences (Assay Ready Control 14,EPI_ISL_710528). The clinical samples were provide by Shuang Ho Hospital, Ministry of Health and Welfare, Republic of China (Taiwan).
Q: Which genes does the Kit target?
A: It covers 2 genes: N and RP genes.
Q: What are the shipment measurements?
A: We want to make sure our kits get to the destination at a cooling temperature, therefore we use Dry Ice and Ice packs in combination.
Q: Can we run the kit in any equipment?
A: Our kit is compatible with RT-qPCR machine standards and our selected one is QuantStudio™ 5 Real-Time PCR System. Please let us know which machine you are using. You are welcome to try the machine of your choice.
| Name | Download |
|---|---|
| PROTOCOL | QP019T-0100_Protocol_20210911.pdf |
| Safety Data Sheet|SDS | QP019T-0100_COVID-19_RT-qPCR_Detection_Kit_Plus_SDS.pdf |