BIO-HELIX - LD003-0500
Novel Green Plus (20000X) DNA Staining Reagent
Size: 500 μl
Novel Green Plus is a next-generation DNA-binding dye which ensures high sensitivity and quality. Compared with Ethidium bromide (EtBr), Novel Green Plus is a much responsive DNA staining reagent under the UV transillumination and is one of the most sensitive stains for detecting dsDNA on the agarose gel. The dye is designed based on the properties relevant to PCR, such as PCR inhibition, stability and fluorescence spectra of the dye. The Novel Green Plus brings a more reliable and safer experience for the user, since the stained gel can be visualized with the blue-light transilluminator, thus avoiding the risk of skin/eye damage as well as reducing the side effects of DNA modification caused by the UV light.
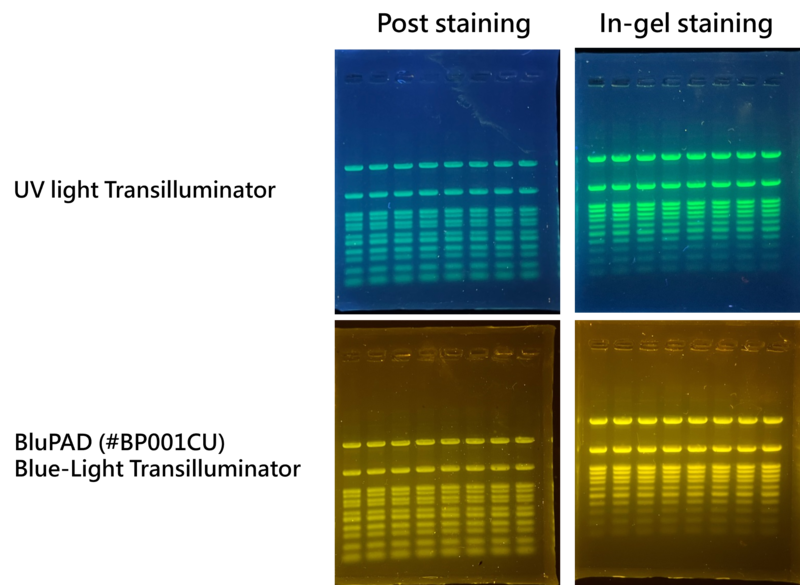
Figure 1. DNA bands detected with Novel Green Plus and both can be detected by UV light and blue light transilluminator. DNA bands were stained with post staining for 20 mins and in-gel staining separately. Each lane was loaded with 5 ul of DM003-R500 in 1% agarose gel.
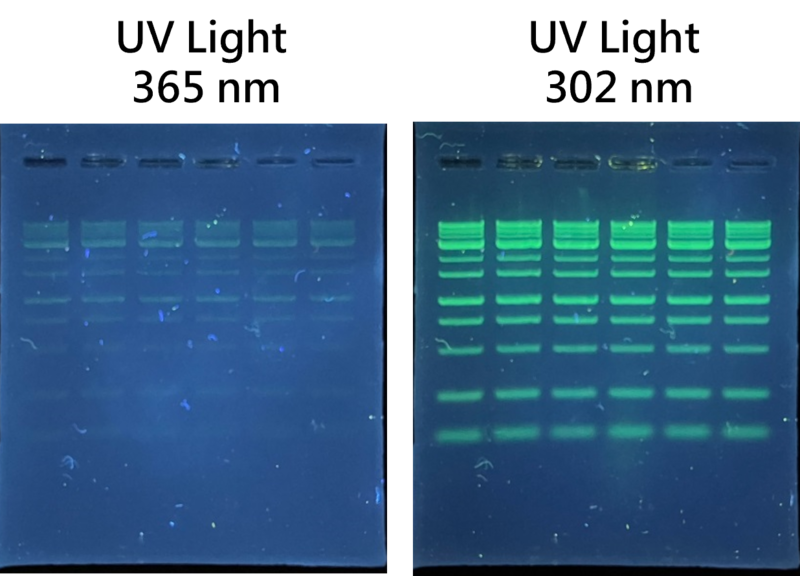
Figure 2. Compare of the detection of DNA bands with post staining with 365 nm and 302 nm in UV light. Each lane was loaded with 5 ul of DM003-R500 in 1% agarose gel and post staining with Novel Green Plus for 20 mins.
* Required materials but not provided & Instrument Compatibility
* Required materials but not provided
- Blue-Light Transilluminator or UV light Transilluminator
- Horizontal Electrophoresis system
- Power supplies
- Microcentrifuge
* Instrument Compatibility
Both Blue-Light and UV transilluminator could detect the signal; broad compatibility range.
* Handling and Disposal
An independent laboratory has shown that Novel Green Plus stain is significantly less mutagenic than the ethidium bromide. However, we must caution that no data are available to address the mutagenicity or toxicity of the Novel Green Plus stain in humans. For the fact that this reagent binds to nucleic acids, it should be treated as a potential mutagen and used with appropriate care. The DMSO stock solution should be handled with particular caution as DMSO is known to facilitate the entry of organic molecules into tissues. Dispose of the stain in compliance with local regulations.
* Important notes
Before opening, the vial should be warmed completely to the ambient temperature for ensuring that the DMSO is thawed thoroughly and that the solution is homogeneous. The DMSO stock solution should be handled with applicable caution because DMSO is known to facilitate the entry of organic molecules into tissues. Dispose of the stain in compliance with local regulations. There is no data addressing the mutagenicity or toxicity of the fluorescent dye in humans. However, the fluorescent dye binds to nucleic acids; it should be recognized as a potential mutagen and used with appropriate care.
》 Post-Electrophoresis DNA Staining
1. Perform electrophoresis on an agarose gel.
The Novel Green Plus is compatible with TAE (40mM Tris-acetate, 1mM EDTA, pH 8), TBE (89 mM Tris base, 89 mM boric acid, 1mM EDTA, pH 8), and TE (20mM Tris base, 1mM EDTA, pH 8) buffers.
2. Dilute the stock Novel Green Plus reagent with the 1:20,000 ratio. Stock stain can be diluted in the TE, TAE or TBE buffer.
If the staining solution is diluted in water, it should be used within 24 hours.
The buffered solution may increase the stability for this fluorescent staining dye.
3. Cover the gel with the staining solution and incubate at the room temperature for 10-30 minutes.
Use a plastic container. Do not use a glass container since it will adsorb much of the dye in the staining solution. Protect the staining container from light by covering it with the aluminum foil or place it in the dark. Agitate the gel gently at the room temperature.
Staining time will vary with the thickness of the gel and the agarose percentage. No destaining is required.
The staining solution may be stored in the dark and at the low temperature for a week or more.
4. Photograph the gel with UV or blue-light transilluminator.
It is important to clean the surface of the transillumuntor after / before each use with the deionized water and a soft cloth. Otherwise, fluorescent dyes may accumulate on the glass surface and cause a high fluorescent background. Video cameras and CCD cameras have a different spectral response than the black and white print film, thus it may not exhibit the same degree of sensitivity.
》 Pre-Electrophoresis DNA Staining / In-Gel DNA Staining
1. Prepare molten agarose gel solution using your standard protocol.
2. Dilute Novel Green Plus 20,000X with the molten gel solution and mix well prior to being poured into the gel container.
Cool the molten agarose gel until it can be handled by hand.
The casted gel with Novel Green Plus Gel Stain will have a slight yellow appearance which is correlated to the dye strength.
Casted gels are stable at 4℃ for 3 days in dark. After three days, the sensitivity will decrease daily.
3. Perform electrophoresis on an agarose gel (avoid light).
The recommended voltage is 4–10 V/cm (distance between the anode and cathode). Avoid using high voltage during electrophoresis. High voltage causes excess heat and affects the dye adversely.
During electrophoresis, the staining dye runs toward the anode, therefore DNA bands with smaller molecular weights may be weaker in intensity due to less staining dye.
4. Imagine the gel with the UV or blue-light transilluminator.
Novel Green Plus is excited at 497 nm but also shows a secondary excitation peak at 248 nm (Fig. 1a). After bound to DNA, the fluorescent emission of the Novel Green Plus is centered at 524 nm (Fig. 1b). These spectral characteristics enable this fluorescent dye to be compatible with a wide variety of gel reading facilities, including UV epi- and transilluminator, argon laser and mercury-arc lamp excitation gel scanners.

Before opening, the vial must be warmed completely to room temperature to ensure that the dimethyl sulfoxide (DMSO) is completely thawed and that the solution is homogeneous. To avoid losing the stain, briefly centrifuge thawed stain in a microfuge to deposit the DMSO solution at the bottom of the vial. The stain may be divided into smaller aliquots and frozen for convenience. Smaller aliquots will thaw more quickly.
| Name | Download |
|---|---|
| PROTOCOL | LD003_Protocol_v4_20240425.pdf |
| Safety Data Sheet|SDS | LD003_0500_SDS_2025.pdf |